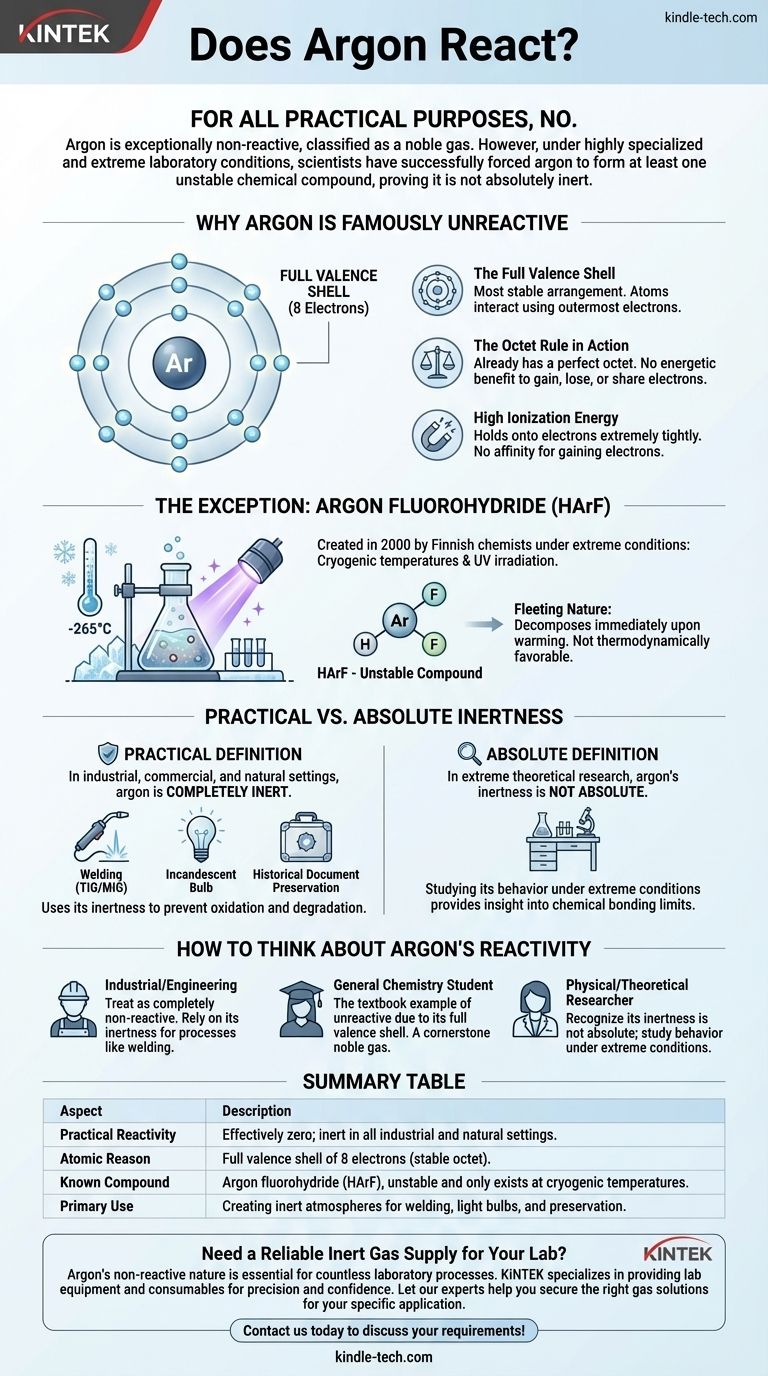
A tutti gli effetti pratici, no. L'argon è eccezionalmente poco reattivo, motivo per cui è classificato come gas nobile. Tuttavia, in condizioni di laboratorio altamente specializzate ed estreme, gli scienziati sono riusciti a forzare l'argon a formare almeno un composto chimico instabile, dimostrando che non è assolutamente inerte.
Il punto chiave è che la "reattività" di un elemento non è una semplice domanda sì/no. Sebbene la configurazione elettronica perfetta dell'argon lo renda inerte in qualsiasi contesto naturale o industriale, la sua non reattività può essere superata con sufficiente energia in condizioni criogeniche, rivelando le sottili complessità del legame chimico.

Perché l'Argon è Famoso per essere "Non Reattivo"
Per capire perché l'argon resiste così fortemente alla formazione di legami chimici, dobbiamo guardare alla sua struttura atomica. La sua reputazione di essere inerte non è arbitraria; è un risultato diretto della sua configurazione elettronica.
Il Guscio di Valenza Completo
Gli atomi interagiscono e formano legami utilizzando i loro elettroni più esterni, noti come elettroni di valenza.
L'argon ha otto elettroni di valenza, che riempiono completamente il suo guscio elettronico esterno. Questa è la disposizione più stabile che un atomo possa avere.
La "Regola dell'Ottetto" in Azione
La "regola dell'ottetto" è un principio fondamentale in chimica che afferma che gli atomi tendono ad acquisire, perdere o condividere elettroni per raggiungere un guscio esterno completo di otto elettroni.
Poiché l'argon possiede già questo ottetto perfetto, non vi è alcun vantaggio energetico nel guadagnare, perdere o condividere elettroni con altri atomi. È già nel suo stato ideale a bassa energia.
Elevata Energia di Ionizzazione
L'energia di ionizzazione è l'energia richiesta per rimuovere un elettrone da un atomo. L'argon ha un'energia di ionizzazione molto elevata, il che significa che trattiene i suoi elettroni molto saldamente.
Allo stesso modo, non ha affinità per acquisire elettroni. Semplicemente non c'è alcun "motivo" chimico per cui l'argon debba impegnarsi in una reazione in circostanze normali.
L'Eccezione che Conferma la Regola
Per decenni, si è creduto che l'argon fosse completamente inerte. Questo è cambiato nel 2000, quando un team di chimici finlandesi ha creato il primo vero composto di argon conosciuto.
Forzare una Reazione in Condizioni Estreme
Il composto, fluoroidruro di argon (HArF), non è stato creato in un normale becher da laboratorio.
Gli scienziati hanno dovuto congelare una miscela di argon e acido fluoridrico su una superficie a temperature prossime allo zero assoluto (circa -265°C o -445°F) e poi irradiarla con una potente luce ultravioletta. Questo apporto energetico estremo è stato sufficiente per forzare temporaneamente l'atomo di argon riluttante a legarsi.
La Natura Fugace dei Composti di Argon
Il composto HArF risultante è incredibilmente instabile. Esiste solo a queste temperature criogeniche.
Se viene riscaldato anche leggermente, i deboli legami si rompono e si decompone immediatamente tornando ad essere argon e acido fluoridrico separati. Ciò evidenzia che il composto non è termodinamicamente favorevole ed esiste solo perché è "intrappolato" dal freddo estremo.
Inerzia Pratica vs. Inerzia Assoluta
Questa scoperta ci costringe a distinguere tra due concetti: ciò che è vero in senso pratico e ciò che è vero in senso assoluto e teorico.
La Definizione Pratica di Inerte
In qualsiasi contesto industriale, commerciale o naturale, l'argon è completamente inerte. Non reagisce con l'aria, l'acqua, i metalli o qualsiasi altra sostanza con cui viene a contatto.
È questa inerzia pratica che lo rende così prezioso.
Perché è Importante per le Applicazioni
La non reattività dell'argon è una caratteristica, non una limitazione. Nella saldatura (TIG/MIG), crea uno "scudo" inerte attorno al metallo fuso, impedendogli di ossidarsi o reagire con i gas presenti nell'aria, garantendo una saldatura pulita e resistente.
Nelle lampadine a incandescenza, un'atmosfera di argon impedisce al filamento di tungsteno caldo di bruciare. Nella conservazione dei documenti storici, fornisce un ambiente privo di ossigeno per arrestare il degrado.
Come Considerare la Reattività dell'Argon
Il tuo contesto determina come dovresti considerare il comportamento chimico dell'argon. Comprendere questa distinzione è fondamentale per applicare correttamente i principi chimici.
- Se lavori in un contesto industriale o ingegneristico: Considera l'argon come un gas completamente non reattivo. La sua inerzia è la sua proprietà più preziosa e ci si può fare affidamento per processi come la saldatura e la produzione.
- Se sei uno studente di chimica generale: Comprendi che l'argon è l'esempio da manuale di elemento non reattivo a causa del suo guscio di elettroni di valenza completo, rendendolo una pietra angolare del gruppo dei gas nobili.
- Se sei un ricercatore in chimica fisica o teorica: Riconosci che l'inerzia dell'argon non è assoluta e studiare il suo comportamento in condizioni estreme fornisce preziose informazioni sui limiti del legame chimico.
In definitiva, l'estrema riluttanza dell'argon a reagire è una proprietà fondamentale che lo rende sia scientificamente interessante sia immensamente utile nel mondo reale.
Tabella Riassuntiva:
| Aspetto | Descrizione |
|---|---|
| Reattività Pratica | Effettivamente zero; inerte in tutte le impostazioni industriali e naturali. |
| Motivazione Atomica | Guscio di valenza completo di 8 elettroni (ottetto stabile). |
| Composto Conosciuto | Fluoroidruro di argon (HArF), instabile ed esistente solo a temperature criogeniche. |
| Uso Principale | Creazione di atmosfere inerti per saldatura, lampadine e conservazione. |
Hai bisogno di una fornitura affidabile di gas inerte per il tuo laboratorio?
La natura non reattiva dell'argon è essenziale per innumerevoli processi di laboratorio, dalla creazione di atmosfere controllate alla manipolazione di materiali sensibili. Garantire una fornitura costante e di elevata purezza è fondamentale per i tuoi risultati.
KINTEK è specializzata nel fornire le attrezzature e i materiali di consumo per laboratorio di cui hai bisogno per operare con precisione e fiducia. Lascia che i nostri esperti ti aiutino a ottenere le giuste soluzioni gassose per la tua specifica applicazione.
Contattaci oggi tramite il nostro modulo per discutere le tue esigenze!
Guida Visiva
Prodotti correlati
- Circolatore refrigerante da 10L, bagno d'acqua di raffreddamento, bagno di reazione a temperatura costante a bassa temperatura
- Collettore di corrente in foglio di alluminio per batteria al litio
- Fornace a Induzione Sottovuoto su Scala di Laboratorio
- Trituratore Ultrafine Vibrante Raffreddato ad Acqua a Bassa Temperatura con Touchscreen
- Produttore Personalizzato di Parti in PTFE Teflon Ciotola per Macinazione
Domande frequenti
- Perché è necessario dotare i sistemi di idrolisi del tutolo di mais di un raffreddamento rapido? Massimizzare la resa di glucosio e xilosio
- Perché è necessario un circolatore di raffreddamento ad alte prestazioni nella desalinizzazione a membrana di silice? Aumenta il trasferimento di massa del permeato
- Perché vengono utilizzate serpentine di raffreddamento interne dopo il trattamento idrotermale? Ottieni rese più elevate nella lavorazione della biomassa
- Perché è necessario un sistema di circolazione di raffreddamento o un refrigeratore per l'SFE? Prevenire il blocco del gas e garantire il flusso ad alta pressione
- Perché è necessario un refrigeratore ad acqua circolante per le nanoparticelle di Blu di Prussia? Garantire stabilità e riproducibilità dei lotti














